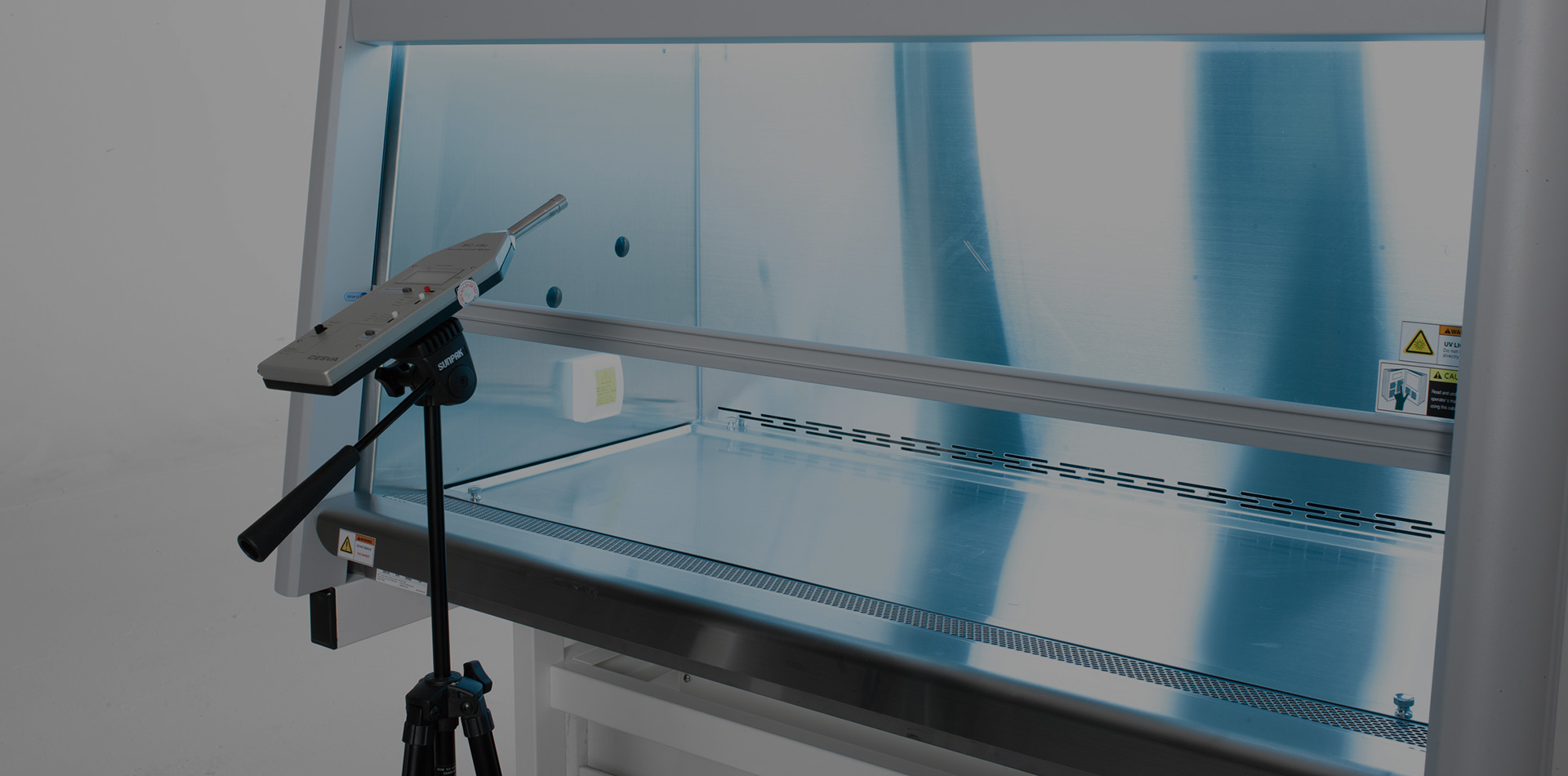
Validation
Validation
CHC Biotech Special Validation
Our unique validation,iq, oq, pq
Validation consists of all processes under IQ, OQ and PQ and it refers to the
verification and documentation of experimental method, process, and equipment in
respect to whether it conforms to the pre-set system settings.
verification and documentation of experimental method, process, and equipment in
respect to whether it conforms to the pre-set system settings.
Q.
OUR QUESTION
Why do we need to do
VALIDATION?
All of us have endless concerns about everything.
How we can reduce the exposure risk of our researchers?
How we can create a safe and clean research environment?
How we can reduce the exposure risk of our researchers?
How we can create a safe and clean research environment?

A.
OUR ANSWER
The answer?
VALIDATION
Researchers are exposed to biohazards and many other
elements while doing experiments. To ensure their safety,
we must first have trustable equipment and facilities. For
the equipment and facility to be trustable, we must first
validate them according to the regulations and guidelines
to make sure they are safe to be used. (Validation is normally done once a year.)
elements while doing experiments. To ensure their safety,
we must first have trustable equipment and facilities. For
the equipment and facility to be trustable, we must first
validate them according to the regulations and guidelines
to make sure they are safe to be used. (Validation is normally done once a year.)

When do we need to conduct validation?
Duringinstallation
After filterexchange
After wemove the equipment
After 1 yearfrom previous validation
When the equipmentis not working as normal
-
Validation service 01
Installation Qualification IQ
- IQ (Installation Qualification) is the process of checking and
verifying the facilities, systems, and equipment on the spot to
determine whether they are manufactured correctly according to the
design specifications and also to verify whether they are installed
properly to perform the desired functions by comparing them with
the requirement standard.
-
Validation service 02
Operational Competency Verification (OQ)
- Operation Qualification (OQ) process is done to
prove and document the ability of each systems and
related machinery to perform respective functions to
maintain the manufacturing process of the factory
-
Validation service 03
Performance Qualification (PQ)
- PQ (Performance Qualification) is a process
conducted on BSC equipment and its related
systems after they pass the OQ process to verify
and document the expected quality and
performance of the product.
Quality Control Test Items
-
Downflow
Velocity Test -
Intake Velocity
(Face Velocity) Test -
HEPA/ULPA
Filter Leak Test -
Airflow smoke
Patterns Test -
Site Installation
Assessment Test -

-
Personnel, Product,
Cross-Contamination
Protection -
Electrical
Test -
Noise/Vibration
Test -
Lighting Intensity
Test -
Particle Count
Test